The first photo ever to capture atoms turning into quantum waves: Schrödinger would be very happy
It opens new horizons in our understanding of the universe, and leads us to explore the invisible world with more powerful tools.
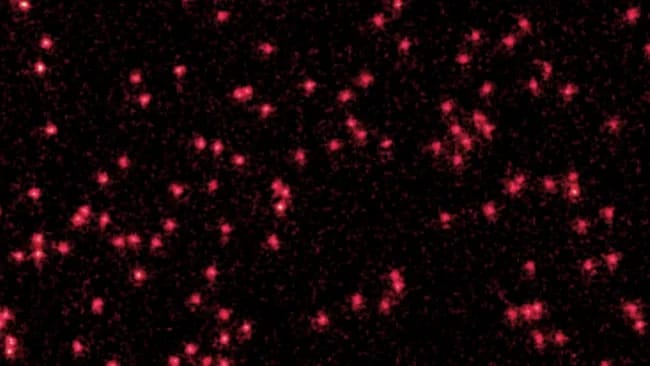
The authors of the study are Drs. Tarik Yefsah, Joris Verstraten, Tim de Jongh, and Bruno Peaudecerf. And the study has not yet been submitted for peer review. The paper is available on the pre-print server ArXiv.: In-situ Imaging of a Single-Atom Wave Packet in Continuous Space
A photo that is going to change the world?
Finally physicists have managed to get a clear image of individual atoms behaving like a wave. This is crucial because the wavelike nature of matter remains one of the most fascinating aspects of QM. To simplify as much as possible, particles can be thought of not as objects with position and momentum, but as quantum fields scattered in space that “collapse” – that is, become real particles – only when an instrument observes them. The Schrödinger equation gives mathematical form to this idea, saying that atoms exist as wave-like probability packets in space: There is no definite position and no definite momentum until we observe it. At the very moment of observation, the wave function collapses into a particle, resulting in the wave-particle duality of the matter.
The image researchers took shows fluorescent lithium atoms turning into small “fuzzy wave packets”. This would prove that atoms exist as both particles and waves, an idea introduced by de Broglie around 1924. A few years after de Broglie’s work, Schrödinger stated that the wave-particle dualism holds true for all quantum particles (not only light). This means that all matter exists both as a particle and as a wave at the same time.
How did they get the image?
Thanks to a new imaging technique that captured frozen lithium atoms. They froze them because in QM the position and momentum cannot be determined before, so freezing them prepared atoms to be observed. Simply put (I’m still trying to figure it out, guys!), physicists first cooled the lithium atoms to temperatures near absolute zero, and then trapped them in an optical lattice. For those of you who have never been in a physics lab, an optical lattice consists of a sheet of glass, with a network of parallel, equal, equidistant lines etched into the surface of the glass at distances comparable to the wavelength of light. It is used to separate the colors of light and exploits precisely the wave nature of it.
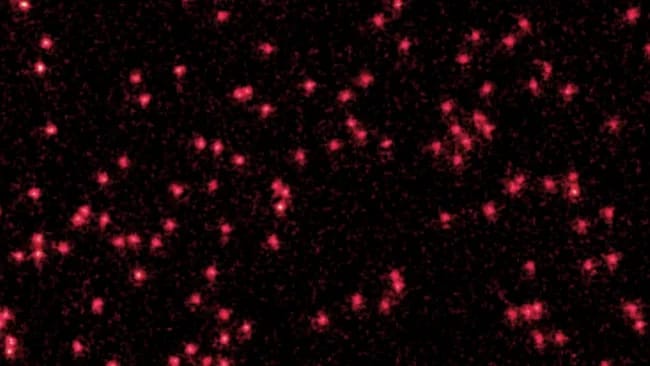
While a microscope camera recorded the light emitted by the atoms, the researchers periodically turned the optical lattice off and on. When the optical lattice is turned on, the state is particle-like; when it is turned off the atomic wave expands. During this process, the trapped atom emits fluorescence that can be detected by the microscopy system. By stitching together multiple images, the researchers were able to capture the moment when individual lithium atoms (optical lattice on) began to behave as localized quantum waves (optical lattice off). So, have a look at the image: Each red dot is a lithium atom in particle form; the fuzzy dots are atoms in the wave state (that is, a cloud of possibilities):
“This imaging method consists in turning back on the lattice to project each wave packet into a single well to turn them into a particle again — it is not a wave anymore,” study co-author Tarik Yefsah, a physicist at the French National Centre for Scientific Research and the École normale supérieure in Paris, told Live Science. “You can see our imaging method as a way to sample the wavefunction density, not unlike the pixels of a CCD camera.” [here]
What will this lead to?
The demonstration of one of the key principles of QM can have a significant impact on the progress of research. In addition, by improving these new techniques, we can better understand the states of matter, open new horizons in our understanding of the universe, and explore the unseen world with more powerful tools.
Guys, this is great!
Thanks for reading! And, please, point out conceptual errors if there are any, so I can correct them! ;-)
More insights
First-Of-Its-Kind Image Shows Single Lithium Atoms Turning Into Quantum Waves
